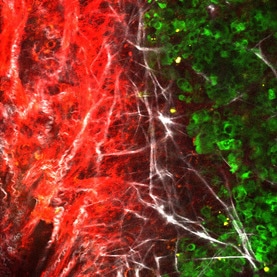
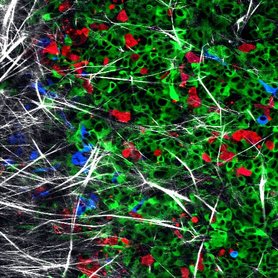
CANCER METASTASIS AND DRUG RESISTANCEThe ECM is a major component of the tumor microenvironment, where it can support cellular growth, promote local invasion from the primary tumor, and contribute to metastatic outgrowth in sites of colonization. The Oudin lab is developing new methods to study the role of the ECM in tumor progression and identify ECM proteins that drive invasion.
While there are several clinical trials ongoing to identify new agents to TNBC, the majority of TNBC patients are treated with anthracycline- or taxane-based chemotherapies in the neoadjuvant setting, followed by surgical resection and adjuvant chemotherapy. While many patients respond well to this approach, as many as 25% will suffer local or metastatic recurrence within five years. Understanding the mechanisms that drive recurrence after chemotherapy treatment is critical to improving survival for patients with TNBC. In addition to triggering death of some cells, chemotherapy induces significant stromal cell recruitment and inflammation. We show that chemotherapy treatment leads to change in the ECM composition of tissues that could contribute to progression and recurrence (Fatherree et al, 2022 Cancer Research).
|
EPILEPSYThe diagnosis of Dr. Oudin’s daughter with SCN8A encephalopathy has led to to a new research area in the lab, investigating how alternative splicing of sodium channels impacts neuronal function and the development of epilepsy. SCN8A is one of the main sodium channels in the brain and it undergoes a developmentally regulated with in splicing which affects function. We aim to develop novel splice-switching antisense oligonucleotide therapies for the treatment of SCN8A neurodevelopmental disorders.
Click here to donate and support SCN8A epilepsy research in the lab. |
CANCER NEUROSCIENCE
|
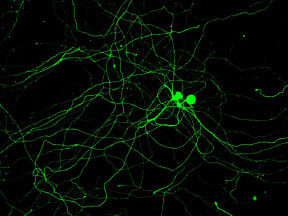
Recently, the presence of peripheral nerves in solid tumors and the upregulation of neuronal genes within tumor cells themselves were both reported to be associated with poor outcome in cancer. Our goal is to use biology, engineering and clinically translatable approaches to understand how neoneurogenesis occurs in tumors, how the neural identity of tumors impacts the systemic regulation of metastasis and how we can leverage this information for non-invasive monitoring and treatment of metastatic disease. We have shown that bioelectricity regulates cancer cell invasion and metastasis which could lead to a new class of therapeutics for patients with metastatic disease (Payne et al., 2022, Ebiomedicine). We have also identified a novel mechanism of sensory nerve-driven invasion in breast cancer via the axon guidance molecule PlexinB3 (Le et al, BiorXiv, 2021).
|
Collaborators
|
At Tufts:
David Kaplan, BME Brian Timko, BME Michael Levin, Biology Andrew Greenberg (TUSM, HNRCA) Stephen Naber, Pathology Chris Dulla, TUSM |
Outside Tufts:
Chris Burge, Biology, MIT |